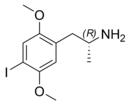
2,5-Dimethoxy-4-iodoamphetamine
DOI
Names
Preferred IUPAC name
1-(4-Iodo-2,5-dimethoxyphenyl)propan-2-amine
Identifiers
ChEMBL
ChemSpider
UNII
InChI=1S/C11H16INO2/c1-7(13)4-8-5-11(15-3)9(12)6-10(8)14-2/h5-7H,4,13H2,1-3H3
Y Key: BGMZUEKZENQUJY-UHFFFAOYSA-N
Y InChI=1/C11H16INO2/c1-7(13)4-8-5-11(15-3)9(12)6-10(8)14-2/h5-7H,4,13H2,1-3H3
Key: BGMZUEKZENQUJY-UHFFFAOYAA
IC(C=C1OC)=C(OC)C=C1CC(C)N
IC(C=C1OC)=C(OC)C=C1C[C@@H](C)N
IC(C=C1OC)=C(OC)C=C1C[C@H](C)N
Properties
C11 H16 INO2
Molar mass
321.1558 g/mol
Melting point
201.5 °C (394.7 °F; 474.6 K) (hydrochloride )
10 mg/mL[ 1]
Pharmacology
Legal status
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
Chemical compound
2,5-Dimethoxy-4-iodoamphetamine (DOI ) is a psychedelic drug of the amphetamine and 4-substituted-2,5-dimethoxyamphetamine (DOx) families.[ 3] [ 4]
DOI acts as a potent serotonin 5-HT2 receptor agonist .[ 3] [ 4] stereocenter and R -(−)-DOI is the more active stereoisomer . In neuroscience research, [125 I] -R -(−)-DOI is used as a radioligand and indicator of the presence of serotonin 5-HT2A receptors .[ 3]
DOI's effects have been compared to LSD , although there are differences that experienced users can distinguish. Besides the longer duration, the trip tends to be more energetic than an LSD trip, with more body load and a different subjective visual experience. The after effects include residual stimulation and difficulty sleeping, which, depending on the dose, may persist for days.[ 5] [ 6]
DOI was first synthesized in 1973, by Coutts and Malicky.[ 3]
Research
Research[ 7] R )-DOI blocks pulmonary inflammation, mucus hyper-production, airway hyper-responsiveness and turns off key genes in in-lung immune response. These effects block the development of allergic asthma in a mouse model.
Several 5-HT2A agonist hallucinogens including (R )-2,5-dimethoxy-4-iodoamphetamine DOI, TCB-2 , LSD and LA-SS-Az have unexpectedly also been found to act as potent inhibitors of TNF , with DOI being the most active, showing TNF inhibition in the picomolar range, an order of magnitude more potent than its action as a hallucinogen.[ 8] [ 9] [ 10]
Pharmacology
DOI is a serotonin 5-HT2A , 5-HT2B and 5-HT2C receptor agonist.[ 4]
DOI has been shown to be an extremely potent inhibitor of tumour necrosis factor-alpha inflammation at picomolar concentrations in cell studies. TNF-alpha is an important target for research into degenerative conditions such as rheumatoid arthritis and Alzheimer's disease , where the disease process involves tissue damage through chronic inflammation . This could make DOI and other 5-HT2A agonists an entirely new area for development of novel treatments for these conditions.[ 12]
DOI has also been shown to induce rapid growth and reorganization of dendritic spines and synaptic connections with other neurons , processes known to underlie neuroplasticity .[ 13]
The drug is not a monoamine releasing agent of serotonin or dopamine .[ 14]
DOI is an agonist of the rat trace amine-associated receptor 1 (TAAR1).[ 15]
History
DOI was first synthesized by Alexander Shulgin .[ 5] radioactive iodine-125 form of DOI for PET imaging was first developed in the lab of David E. Nichols .
In January 2007, British police reported that three young men had fallen ill, reportedly, after taking DOI at a rave in Biggleswade , near Milton Keynes , and warned others who had taken it to seek medical attention. This would appear to be the first indication that DOI has found more widespread use as a recreational drug in the UK.[ 16]
South Australian man Cody Edwards who brutally murdered Synamin Bell controversially plead guilty to the lesser sentence of manslaughter after attesting that the drug DOI had induced paranoia , and that he had subsequently acted in ‘self-defence ’ when he had beaten the mother-of-three to death with a dumbbell, resulting in over fifty wounds.[ 17]
Legal status
Australia
The Standard for the Uniform Scheduling of Medicines and Poisons (SUSMP) of Australia does not list DOI as a prohibited substance.[ 18]
Canada
Listed as a Schedule 1 [ 19] [ 20] Safe Streets and Communities Act ,Schedule 3 to Schedule 1 .[ 21]
Denmark
Illegal since 8 April 2007.[ 22]
Finland
DOI is classified as a psychoactive substance banned from the consumer market in Finland.[ 23]
Sweden
Sveriges riksdag "substances, plant materials and fungi which normally do not have medical use" ) as narcotics in Sweden as of August 30, 2007, published by Medical Products Agency LVFS 2007:10 listed as DOI, 4-jod-2,5-dimetoxi-amfetamin .[ 24]
United States
As of 2023, DOI is not scheduled in the United States,[ 25] DOB ), in which case, sales or possession could be prosecuted under the Federal Analogue Act . In December 2023, the US Drug Enforcement Administration issued a notice of proposed rulemaking that would classify both 2,5-dimethoxy-4-iodoamphetamine and 2,5-dimethoxy-4-chloroamphetamine as schedule I controlled substances.[ 26]
DOI is regularly used in animal and in vitro research.[ 27] [ 26]
US State of Florida
DOI is a Schedule I controlled substance in the state of Florida.[ 28]
See also
References
^ "D101 DOI hydrochloride ≥98% (HPLC), solid" . Retrieved 13 April 2008 .^ Anvisa (2023-07-24). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27 .^ a b c d Glennon RA, Dukat M (June 2024). "1-(2,5-Dimethoxy-4-iodophenyl)-2-aminopropane (DOI): From an Obscure to Pivotal Member of the DOX Family of Serotonergic Psychedelic Agents - A Review" . ACS Pharmacol Transl Sci . 7 (6): 1722– 1745. doi :10.1021/acsptsci.4c00157 PMID 38898956 . ^ a b c d Canal, CE; Morgan, D (July 2012). "Head-twitch response in rodents induced by the hallucinogen 2,5-dimethoxy-4-iodoamphetamine: a comprehensive history, a re-evaluation of mechanisms, and its utility as a model" . Drug Testing and Analysis . 4 (7– 8): 556– 576. doi :10.1002/dta.1333 . PMC 3722587 PMID 22517680 . ^ a b Shulgin, A; Shulgin, A (1990). "#67 DOI" . PiHKAL: A Chemical Love Story . Transform Press. ISBN 978-0963009609 Archived from the original on 2014-10-27. Retrieved 2014-12-17 . ^ "LSD Blotter Acid Mimics (Actually containing 4-IODO-2,5-DIMETHOXYAMPHETAMINE (DOI) and 4-CHLORO-2,5-DIMETHOXYAMPHETAMINE (DOC)) in Lantana, Florida" . DEA Microgram Bulletin . Washington, DC: Office of Forensic Sciences, Drug Enforcement Administration. June 2008. Archived from the original on 2009-02-04. Retrieved 12 February 2009 .^ "LSU Health New Orleans research finds psychedelic drug prevents asthma development in mice" . EurekAlert! .^ Miller KJ, Gonzalez HA (December 1998). "Serotonin 5-HT2A receptor activation inhibits cytokine-stimulated inducible nitric oxide synthase in C6 glioma cells". Ann. N. Y. Acad. Sci . 861 (1): 169– 73. Bibcode :1998NYASA.861..169M . doi :10.1111/j.1749-6632.1998.tb10188.x . PMID 9928254 . S2CID 23264746 . ^ Yu B, Becnel J, Zerfaoui M, Rohatgi R, Boulares AH, Nichols CD (November 2008). "Serotonin 5-hydroxytryptamine(2A) receptor activation suppresses tumor necrosis factor-alpha-induced inflammation with extraordinary potency". J. Pharmacol. Exp. Ther . 327 (2): 316– 23. doi :10.1124/jpet.108.143461 . PMID 18708586 . S2CID 25374241 . ^ Pelletier M, Siegel RM (December 2009). "Wishing away inflammation? New links between serotonin and TNF signaling" . Mol. Interv . 9 (6): 299– 301. doi :10.1124/mi.9.6.5 . PMC 2861806 PMID 20048135 . ^ a b c Roth, BL ; Driscol, J (12 January 2011). "PDSP Ki Database" . Psychoactive Drug Screening Program (PDSP) . University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Archived from the original on 8 November 2013. Retrieved 4 March 2014 .^ Yu, B; Becnel, J; Zerfaoui, M; Rohatgi, R; Boulares, AH; Nichols, CD (2008). "Serotonin 5-Hydroxytryptamine2A Receptor Activation Suppresses Tumor Necrosis Factor-α-Induced Inflammation with Extraordinary Potency". Journal of Pharmacology and Experimental Therapeutics . 327 (2): 316– 323. doi :10.1124/jpet.108.143461 . PMID 18708586 . S2CID 25374241 . ^ Jones, KA; Srivastava, DP; Allen, JA; Strachan, RT; Roth, BL; Penzes, P (17 November 2009). "Rapid modulation of spine morphology by the 5-HT2A serotonin receptor through kalirin-7 signaling" . Proceedings of the National Academy of Sciences . 106 (46): 19575– 19580. Bibcode :2009PNAS..10619575J . doi :10.1073/pnas.0905884106 PMC 2780750 PMID 19889983 . ^ Matsumoto T, Maeno Y, Kato H, Seko-Nakamura Y, Monma-Ohtaki J, Ishiba A, Nagao M, Aoki Y (August 2014). "5-hydroxytryptamine- and dopamine-releasing effects of ring-substituted amphetamines on rat brain: a comparative study using in vivo microdialysis". Eur Neuropsychopharmacol . 24 (8): 1362– 1370. doi :10.1016/j.euroneuro.2014.04.009 . PMID 24862256 . ^ Bunzow JR, Sonders MS, Arttamangkul S, Harrison LM, Zhang G, Quigley DI, Darland T, Suchland KL, Pasumamula S, Kennedy JL, Olson SB, Magenis RE, Amara SG, Grandy DK (December 2001). "Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor". Mol Pharmacol . 60 (6): 1181– 1188. doi :10.1124/mol.60.6.1181 . PMID 11723224 . ^ "New drug alert as three taken ill" . BBC News ^ "Man sentenced to prison for manslaughter of partner, as government moves to close 'defence loophole' - ABC News" . amp.abc.net.au . Retrieved 2024-09-06 .^ Gill, A (22 July 2013). "POISONS STANDARD 2013" (PDF) . Therapeutic Goods Administration. Australian Government Department of Health and Ageing. Retrieved 4 March 2014 . ^ "Controlled Drugs and Substances Act : Legislative history · Schedule I · Section 19: Tramadol [Proposed]; Amphetamines" . isomerdesign.com . Archived from the original on 2022-03-31. Retrieved 2012-11-27 .^ "Controlled Drugs and Substances Act : Definitions and Interpretations" . isomerdesign.com . Archived from the original on 2013-11-10. Retrieved 2012-11-27 .^ [1] (in English) ^ "Erowid DOC Vault : Legal Status" . www.erowid.org .^ "FINLEX ® - Ajantasainen lainsäädäntö: Valtioneuvoston asetus kuluttajamarkkinoilta… 1130/2014" .^ "Läkemedelsverkets föreskrifter - LVFS och HSLF-FS | Läkemedelsverket" (PDF) .^ "PART 1308 - Section 1308.11 Schedule I" . www.deadiversion.usdoj.gov . Archived from the original on 2009-08-27. Retrieved 2014-12-17 .^ a b "Schedules of Controlled Substances: Placement of 2,5-dimethoxy-4-iodoamphetamine (DOI) and 2,5-dimethoxy-4-chloroamphetamine (DOC) in Schedule I" . www.regulations.gov .^ Mario de la Fuente Revenga; Bohan Zhu; Christopher A. Guevara; George W. Huntley; Chang Lu; Javier González-Maeso (2021). "Prolonged epigenomic and synaptic plasticity alterations following single exposure to a psychedelic in mice" . Cell Reports . 37 (3): 109836. doi :10.1016/j.celrep.2021.109836 . PMC 8582597 PMID 34686347 . ^ "Statutes & Constitution :View Statutes : Online Sunshine" . leg.state.fl.us .
External links
Psychedelics (5-HT2A
Benzofurans Lyserg‐ Phenethyl‐
2C-x
3C-x 4C-x DOx HOT-x MDxx Mescaline (subst.) TMAs
TMA
TMA-2
TMA-3
TMA-4
TMA-5
TMA-6 Others
Piperazines Tryptamines
alpha -alkyltryptaminesx -DALT x -DET x -DiPT x -DMT
4,5-DHP-DMT 2,N,N-TMT 4-AcO-DMT 4-HO-5-MeO-DMT 4,N,N-TMT 4-Propionyloxy-DMT 5,6-diBr-DMT 5-AcO-DMT 5-Bromo-DMT 5-MeO-2,N ,N -TMT 5-MeO-4,N ,N -TMT 5-MeO-α,N,N-TMT 5-MeO-DMT 5-N ,N -TMT 7,N,N-TMT α,N,N-TMT (Bufotenin) 5-HO-DMT DMT Norbaeocystin (Psilocin) 4-HO-DMT (Psilocybin) 4-PO-DMT x -DPT Ibogaine-related x -MET x -MiPT Others
Others
Dissociatives (NMDAR antagonists )
Deliriants (mAChR antagonists ) Others
5-HT1
5-HT1A
Agonists: 8-OH-DPAT Adatanserin Amphetamine Antidepressants (e.g., etoperidone , hydroxynefazodone , nefazodone , trazodone , triazoledione , vilazodone , vortioxetine )Atypical antipsychotics (e.g., aripiprazole , asenapine , brexpiprazole , cariprazine , clozapine , lurasidone , quetiapine , ziprasidone )Azapirones (e.g., buspirone , eptapirone , gepirone , perospirone , tandospirone )Bay R 1531 Befiradol BMY-14802 Cannabidiol Dimemebfe Dopamine Ebalzotan Eltoprazine Enciprazine Ergolines (e.g., bromocriptine , cabergoline , dihydroergotamine , ergotamine , lisuride , LSD , methylergometrine (methylergonovine) , methysergide , pergolide )F-11,461 F-12826 F-13714 F-14679 F-15063 F-15,599 Flesinoxan Flibanserin Flumexadol Hypidone Lesopitron LY-293284 LY-301317 mCPP MKC-242 Naluzotan NBUMP Osemozotan Oxaflozane Pardoprunox Piclozotan Rauwolscine Repinotan Roxindole RU-24,969 S-14,506 S-14671 S-15535 Sarizotan Serotonin (5-HT) SSR-181507 Sunepitron Tryptamines (e.g., 5-CT , 5-MeO-DMT , 5-MT , bufotenin , DMT , indorenate , N-Me-5-HT , psilocin , psilocybin )TGBA01AD U-92,016-A Urapidil Vilazodone Xaliproden Yohimbine
Antagonists: Atypical antipsychotics (e.g., iloperidone , risperidone , sertindole )AV965 Beta blockers (e.g., alprenolol , carteolol , cyanopindolol , iodocyanopindolol , isamoltane , oxprenolol , penbutolol , pindobind , pindolol , propranolol , tertatolol )BMY-7,378 CSP-2503 Dotarizine Ergolines (e.g., metergoline )FCE-24379 Flopropione GR-46611 Isamoltane Lecozotan Mefway Metitepine (methiothepin) MIN-117 (WF-516) MPPF NAN-190 Robalzotan S-15535 SB-649,915 SDZ 216-525 Spiperone Spiramide Spiroxatrine UH-301 WAY-100135 WAY-100635 Xylamidine
5-HT1B
Agonists: Anpirtoline CGS-12066A CP-93129 CP-94253 CP-122,288 CP-135807 Eltoprazine Ergolines (e.g., bromocriptine , dihydroergotamine , ergotamine , methylergometrine (methylergonovine) , methysergide , pergolide )mCPP RU-24,969 Serotonin (5-HT) Triptans (e.g., avitriptan , donitriptan , eletriptan , sumatriptan , zolmitriptan )TFMPP Tryptamines (e.g., 5-BT , 5-CT , 5-MT , DMT )Vortioxetine
5-HT1D
Agonists: CP-122,288 CP-135807 CP-286601 Ergolines (e.g., bromocriptine , cabergoline , dihydroergotamine , ergotamine , LSD , methysergide )GR-46611 L-694247 L-772405 mCPP PNU-109291 PNU-142633 Serotonin (5-HT) TGBA01AD Triptans (e.g., almotriptan , avitriptan , donitriptan , eletriptan , frovatriptan , naratriptan , rizatriptan , sumatriptan , zolmitriptan )Tryptamines (e.g., 5-BT , 5-CT , 5-Et-DMT , 5-MT , 5-(nonyloxy)tryptamine , DMT )
5-HT1E
5-HT1F
5-HT2
5-HT2A
Agonists: 25H/NB series (e.g., 25I-NBF , 25I-NBMD , 25I-NBOH , 25I-NBOMe , 25B-NBOMe , 25C-NBOMe , 25TFM-NBOMe , 2CBCB-NBOMe , 25CN-NBOH , 2CBFly-NBOMe )2Cs (e.g., 2C-B , 2C-E , 2C-I , 2C-T-2 , 2C-T-7 , 2C-T-21 )2C-B-FLY 2CB-Ind 5-Methoxytryptamines (5-MeO-DET , 5-MeO-DiPT , 5-MeO-DMT , 5-MeO-DPT , 5-MT )α-Alkyltryptamines (e.g., 5-Cl-αMT , 5-Fl-αMT , 5-MeO-αET , 5-MeO-αMT , α-Me-5-HT , αET , αMT )AL-34662 AL-37350A Bromo-DragonFLY Dimemebfe DMBMPP DOx (e.g., DOB , DOC , DOI , DOM )Efavirenz Ergolines (e.g., 1P-LSD , ALD-52 , bromocriptine , cabergoline , ergine (LSA) , ergometrine (ergonovine) , ergotamine , lisuride , LA-SS-Az , LSB , LSD , LSD-Pip , LSH , LSP , methylergometrine (methylergonovine) , pergolide )Flumexadol IHCH-7113 Jimscaline Lorcaserin MDxx (e.g., MDA (tenamfetamine) , MDMA (midomafetamine) , MDOH , MMDA )O-4310 Oxaflozane PHA-57378 PNU-22394 PNU-181731 RH-34 SCHEMBL5334361 Phenethylamines (e.g., lophophine , mescaline )Piperazines (e.g., BZP , quipazine , TFMPP )Serotonin (5-HT) TCB-2 TFMFly Tryptamines (e.g., 5-BT , 5-CT , bufotenin , DET , DiPT , DMT , DPT , psilocin , psilocybin , tryptamine )
Antagonists: 5-I-R91150 5-MeO-NBpBrT AC-90179 Adatanserin Altanserin Antihistamines (e.g., cyproheptadine , hydroxyzine , ketotifen , perlapine )AMDA Atypical antipsychotics (e.g., amperozide , aripiprazole , asenapine , blonanserin , brexpiprazole , carpipramine , clocapramine , clorotepine , clozapine , fluperlapine , gevotroline , iloperidone , lurasidone , melperone , mosapramine , ocaperidone , olanzapine , paliperidone , quetiapine , risperidone , sertindole , zicronapine , ziprasidone , zotepine )Chlorprothixene Cinanserin CSP-2503 Deramciclane Dotarizine Eplivanserin Ergolines (e.g., amesergide , LY-53857 , LY-215,840 , mesulergine , metergoline , methysergide , sergolexole )Fananserin Flibanserin Glemanserin Irindalone Ketanserin KML-010 Landipirdine LY-393558 mCPP Medifoxamine Metitepine (methiothepin) MIN-117 (WF-516) Naftidrofuryl Nantenine Nelotanserin Opiranserin (VVZ-149) Pelanserin Phenoxybenzamine Pimavanserin Pirenperone Pizotifen Pruvanserin Rauwolscine Ritanserin Roluperidone S-14671 Sarpogrelate Serotonin antagonists and reuptake inhibitors (e.g., etoperidone , hydroxynefazodone , lubazodone , mepiprazole , nefazodone , triazoledione , trazodone )SR-46349B TGBA01AD Teniloxazine Temanogrel Tetracyclic antidepressants (e.g., amoxapine , aptazapine , esmirtazapine , maprotiline , mianserin , mirtazapine )Tricyclic antidepressants (e.g., amitriptyline )Typical antipsychotics (e.g., chlorpromazine , fluphenazine , haloperidol , loxapine , perphenazine , pimozide , pipamperone , prochlorperazine , setoperone , spiperone , spiramide , thioridazine , thiothixene , trifluoperazine )Volinanserin Xylamidine Yohimbine
5-HT2B
Agonists: 4-Methylaminorex Aminorex Amphetamines (e.g., chlorphentermine , cloforex , dexfenfluramine , fenfluramine , levofenfluramine , norfenfluramine )BW-723C86 DOx (e.g., DOB , DOC , DOI , DOM )Ergolines (e.g., cabergoline , dihydroergocryptine , dihydroergotamine , ergotamine , methylergometrine (methylergonovine) , methysergide , pergolide )Lorcaserin MDxx (e.g., MDA (tenamfetamine) , MDMA (midomafetamine) , MDOH , MMDA )Piperazines (e.g., TFMPP )PNU-22394 Ro60-0175 Serotonin (5-HT) Tryptamines (e.g., 5-BT , 5-CT , 5-MT , α-Me-5-HT , bufotenin , DET , DiPT , DMT , DPT , psilocin , psilocybin , tryptamine )
Antagonists: Agomelatine Atypical antipsychotics (e.g., amisulpride , aripiprazole , asenapine , brexpiprazole , cariprazine , clozapine , N-desalkylquetiapine (norquetiapine) , N-desmethylclozapine (norclozapine) , olanzapine , pipamperone , quetiapine , risperidone , ziprasidone )Cyproheptadine EGIS-7625 Ergolines (e.g., amesergide , bromocriptine , lisuride , LY-53857 , LY-272015 , mesulergine )Ketanserin LY-393558 mCPP Metadoxine Metitepine (methiothepin) Pirenperone Pizotifen Propranolol PRX-08066 Rauwolscine Ritanserin RS-127445 Sarpogrelate SB-200646 SB-204741 SB-206553 SB-215505 SB-221284 SB-228357 SDZ SER-082 Tegaserod Tetracyclic antidepressants (e.g., amoxapine , mianserin , mirtazapine )Trazodone Typical antipsychotics (e.g., chlorpromazine )TIK-301 Yohimbine
5-HT2C
Agonists: 2Cs (e.g., 2C-B , 2C-E , 2C-I , 2C-T-2 , 2C-T-7 , 2C-T-21 )5-Methoxytryptamines (5-MeO-DET , 5-MeO-DiPT , 5-MeO-DMT , 5-MeO-DPT , 5-MT )α-Alkyltryptamines (e.g., 5-Cl-αMT , 5-Fl-αMT , 5-MeO-αET , 5-MeO-αMT , α-Me-5-HT , αET , αMT )A-372159 AL-38022A Alstonine CP-809101 Dimemebfe DOx (e.g., DOB , DOC , DOI , DOM )Ergolines (e.g., ALD-52 , cabergoline , dihydroergotamine , ergine (LSA) , ergotamine , lisuride , LA-SS-Az , LSB , LSD , LSD-Pip , LSH , LSP , pergolide )Flumexadol Lorcaserin MDxx (e.g., MDA (tenamfetamine) , MDMA (midomafetamine) , MDOH , MMDA )MK-212 ORG-12962 ORG-37684 Oxaflozane PHA-57378 Phenethylamines (e.g., lophophine , mescaline )Piperazines (e.g., aripiprazole , BZP , mCPP , quipazine , TFMPP )PNU-22394 PNU-181731 Ro60-0175 Ro60-0213 Serotonin (5-HT) Tryptamines (e.g., 5-BT , 5-CT , bufotenin , DET , DiPT , DMT , DPT , psilocin , psilocybin , tryptamine )Vabicaserin WAY-629 WAY-161503 YM-348
Antagonists: Adatanserin Agomelatine Atypical antipsychotics (e.g., asenapine , clorotepine , clozapine , fluperlapine , iloperidone , melperone , olanzapine , paliperidone , quetiapine , risperidone , sertindole , ziprasidone , zotepine )Captodiame CEPC Cinanserin Cyproheptadine Deramciclane Desmetramadol Dotarizine Eltoprazine Ergolines (e.g., amesergide , bromocriptine , LY-53857 , LY-215,840 , mesulergine , metergoline , methysergide , sergolexole )Etoperidone Fluoxetine FR-260010 Irindalone Ketanserin Ketotifen Latrepirdine (dimebolin) Medifoxamine Metitepine (methiothepin) Nefazodone Pirenperone Pizotifen Propranolol Ritanserin RS-102221 S-14671 SB-200646 SB-206553 SB-221284 SB-228357 SB-242084 SB-243213 SDZ SER-082 Tedatioxetine Tetracyclic antidepressants (e.g., amoxapine , aptazapine , esmirtazapine , maprotiline , mianserin , mirtazapine )TIK-301 Tramadol Trazodone Tricyclic antidepressants (e.g., amitriptyline , nortriptyline )Typical antipsychotics (e.g., chlorpromazine , loxapine , pimozide , pipamperone , thioridazine )Xylamidine
5-HT3 –7
5-HT3
Agonists: Alcohols (e.g., butanol , ethanol (alcohol) , trichloroethanol )m-CPBG Phenylbiguanide Piperazines (e.g., BZP , mCPP , quipazine )RS-56812 Serotonin (5-HT) SR-57227 SR-57227A Tryptamines (e.g., 2-Me-5-HT , 5-CT , bufotenidine (5-HTQ) )Volatiles/gases (e.g., halothane , isoflurane , toluene , trichloroethane )YM-31636
Antagonists: Alosetron Anpirtoline Arazasetron AS-8112 Atypical antipsychotics (e.g., clozapine , olanzapine , quetiapine )Azasetron Batanopride Bemesetron (MDL-72222) Bupropion Cilansetron CSP-2503 Dazopride Dolasetron Galanolactone Granisetron Hydroxybupropion Lerisetron Memantine Ondansetron Palonosetron Ramosetron Renzapride Ricasetron Tedatioxetine Tetracyclic antidepressants (e.g., amoxapine , mianserin , mirtazapine )Thujone Tropanserin Tropisetron Typical antipsychotics (e.g., loxapine )Volatiles/gases (e.g., nitrous oxide , sevoflurane , xenon )Vortioxetine Zacopride Zatosetron
5-HT4
5-HT5A
5-HT6
Agonists: Ergolines (e.g., dihydroergocryptine , dihydroergotamine , ergotamine , lisuride , LSD , mesulergine , metergoline , methysergide )Hypidone Serotonin (5-HT) Tryptamines (e.g., 2-Me-5-HT , 5-BT , 5-CT , 5-MT , Bufotenin , E-6801 , E-6837 , EMD-386088 , EMDT , LY-586713 , N-Me-5-HT , ST-1936 , tryptamine )WAY-181187 WAY-208466
Antagonists: ABT-354 Atypical antipsychotics (e.g., aripiprazole , asenapine , clorotepine , clozapine , fluperlapine , iloperidone , olanzapine , tiospirone )AVN-101 AVN-211 AVN-322 AVN-397 BGC20-760 BVT-5182 BVT-74316 Cerlapirdine EGIS-12,233 GW-742457 Idalopirdine Ketanserin Landipirdine Latrepirdine (dimebolin) Masupirdine Metitepine (methiothepin) MS-245 PRX-07034 Ritanserin Ro 04-6790 Ro 63-0563 SB-258585 SB-271046 SB-357134 SB-399885 SB-742457 Tetracyclic antidepressants (e.g., amoxapine , mianserin )Tricyclic antidepressants (e.g., amitriptyline , clomipramine , doxepin , nortriptyline )Typical antipsychotics (e.g., chlorpromazine , loxapine )
5-HT7
Antagonists: Atypical antipsychotics (e.g., amisulpride , aripiprazole , asenapine , brexpiprazole , clorotepine , clozapine , fluperlapine , olanzapine , risperidone , sertindole , tiospirone , ziprasidone , zotepine )Butaclamol DR-4485 EGIS-12,233 Ergolines (e.g., 2-Br-LSD (BOL-148) , amesergide , bromocriptine , cabergoline , dihydroergotamine , ergotamine , LY-53857 , LY-215,840 , mesulergine , metergoline , methysergide , sergolexole )JNJ-18038683 Ketanserin LY-215,840 Metitepine (methiothepin) Ritanserin SB-258719 SB-258741 SB-269970 SB-656104 SB-656104A SB-691673 SLV-313 SLV-314 Spiperone SSR-181507 Tetracyclic antidepressants (e.g., amoxapine , maprotiline , mianserin , mirtazapine )Tricyclic antidepressants (e.g., amitriptyline , clomipramine , imipramine )Typical antipsychotics (e.g., acetophenazine , chlorpromazine , chlorprothixene , fluphenazine , loxapine , pimozide )Vortioxetine
TAAR1 Tooltip Trace amine-associated receptor 1
TAAR5 Tooltip Trace amine-associated receptor 5
Notes: List of trace amines , TAAR , and TAAR1 pages. See also: Receptor/signaling modulators