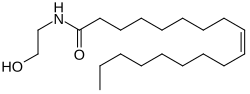
Oleoylethanolamide
Names
Preferred IUPAC name
(9Z )-N -(2-Hydroxyethyl)octadec-9-enamide
Identifiers
ChEBI
ChemSpider
ECHA InfoCard 100.003.532
UNII
InChI=1S/C20H39NO2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-20(23)21-18-19-22/h9-10,22H,2-8,11-19H2,1H3,(H,21,23)/b10-9-
N Key: BOWVQLFMWHZBEF-KTKRTIGZSA-N
N InChI=1/C20H39NO2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-20(23)21-18-19-22/h9-10,22H,2-8,11-19H2,1H3,(H,21,23)/b10-9-
Key: BOWVQLFMWHZBEF-KTKRTIGZBW
CCCCCCCC\C=C/CCCCCCCC(=O)NCCO
Properties
C 20 H 39 N O 2
Molar mass
−1
Appearance
White solid
Melting point
59–60 °C (138–140 °F; 332–333 K)
Solubility in ethanol and DMSO
Soluble
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
Chemical compound
Oleoylethanolamide (OEA ) is an endogenous peroxisome proliferator-activated receptor alpha (PPAR -α) agonist . It is a naturally occurring ethanolamide lipid that regulates feeding and body weight in vertebrates ranging from mice to pythons.[ 1] [ 2] [ 3]
OEA is a shorter, monounsaturated analogue of the endocannabinoid anandamide , but unlike anandamide it acts independently of the cannabinoid pathway, regulating PPAR-α activity to stimulate lipolysis .[ 4]
OEA is produced by the small intestine following feeding in two steps. First an N -acyl transferasephosphatidylethanolamine (PE) to the oleoyl group (one variety of acyl group) derived from sn -1-oleoyl-phosphatidylcholine, which contains the fatty acid oleic acid at the sn-1 position.[ 5] N -acylphosphatidylethanolaminehydrolyzed ) by N -acyl phosphatidylethanolamine-specific phospholipase Dphosphatidic acid and OEA. The biosynthesis of OEA and other bioactive lipid amides is modulated by bile acids .[ 6]
OEA has been demonstrated to bind to the novel cannabinoid receptor GPR119 .[ 7] [ 8]
OEA has been hypothesized to play a key role in the inhibition of food seeking behavior and in the lipolysis of brown bears "ursus arctos " during the hibernation season together with the alteration of the endocannabinoid system required for the metabolic changes for hibernation.[ 9]
OEA has been reported to lengthen the life span of the roundworm Caenorhabditis elegans [ 10]
OEA is mainly known by its anorexigenic effects. However, it has also neuroprotective properties. In this sense, recent research has demonstrated that OEA reduces neuronal death in a murine model of aggressive neurodegeneration.[ 11] [ 12]
References
^ Gaetani S, Oveisi F, Piomelli D (2003). "Modulation of meal pattern in the rat by the anorexic lipid mediator oleoylethanolamine" . Neuropsychopharmacology . 28 (7): 1311– 6. doi :10.1038/sj.npp.1300166 PMID 12700681 . ^ Lo Verme J, Gaetani S, Fu J, Oveisi F, Burton K, Piomelli D (2005). "Regulation of food intake by oleoylethanolamine" . Cell. Mol. Life Sci . 62 (6): 708– 16. doi :10.1007/s00018-004-4494-0 . PMID 15770421 . S2CID 26838764 . ^ Giuseppe Astarita; Bryan C. Rourke; Johnnie B. Andersen; Jin Fu; Janet H. Kim; Albert F. Bennett; James W. Hicks & Daniele Piomelli (2005-12-22). "Postprandial increase of oleoylethanolamine mobilization in small intestine of the Burmese python (Python molurus)" . Am J Physiol Regul Integr Comp Physiol . 290 (5): R1407 – R1412 . doi :10.1152/ajpregu.00664.2005 . PMID 16373434 . S2CID 11852711 . ^ Gaetani S, Kaye WH, Cuomo V, Piomelli D (September 2008). "Role of endocannabinoids and their analogues in obesity and eating disorders" . Eat Weight Disord . 13 (3): e42–8. PMID 19011363 . ^ illustration ^ Magotti P, Bauer I, Igarashi M, Babagoli M, Marotta R, Piomelli D, Garau G (Dec 2014). "Structure of Human N-Acylphosphatidylethanolamine-Hydrolyzing Phospholipase D: Regulation of Fatty Acid Ethanolamide Biosynthesis by Bile Acids" . Structure . 23 (3): 598– 604. doi :10.1016/j.str.2014.12.018 . PMC 4351732 PMID 25684574 . ^ Overton HA, Babbs AJ, Doel SM, Fyfe MC, Gardner LS, Griffin G, Jackson HC, Procter MJ, Rasamison CM, Tang-Christensen M, Widdowson PS, Williams GM, Reynet C (2006). "Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents" . Cell Metab . 3 (3): 167– 175. doi :10.1016/j.cmet.2006.02.004 PMID 16517404 . ^ Brown AJ. (2007). "Novel cannabinoid receptors" . Br J Pharmacol . 152 (5): 567– 575. doi :10.1038/sj.bjp.0707481 . PMC 2190013 PMID 17906678 . ^ Boyer C, Cussonneau L, Brun C, Deval C, Pais de Barros JP, Chanon S, Bernoud-Hubac N, Daira P, Evans AL, Arnemo JM, Swenson JE, Gauquelin-Koch G, Simon C, Blanc S, Combaret L, Bertile F, Lefai E (2020). "Specific shifts in the endocannabinoid system in hibernating brown bears" . Frontiers in Zoology . 17 (1): 35. doi :10.1186/s12983-020-00380-y PMC 7681968 PMID 33292302 . ^ Folick A, Oakley HD, Yu Y, Armstrong EH, Kumari M, Sanor L, Moore DD, Ortlund EA, Zechner R, Wang MC (2015). "Aging. Lysosomal signaling molecules regulate longevity in Caenorhabditis elegans" . Science . 347 (6217): 83– 6. doi :10.1126/science.1258857 . PMC 4425353 PMID 25554789 . ^ Pérez-Martín, Ester; Muñoz-Castañeda, Rodrigo; Moutin, Marie-Jo; Ávila-Zarza, Carmelo A.; Muñoz-Castañeda, José M.; Del Pilar, Carlos; Alonso, José R.; Andrieux, Annie; Díaz, David; Weruaga, Eduardo (July 2021). "Oleoylethanolamide Delays the Dysfunction and Death of Purkinje Cells and Ameliorates Behavioral Defects in a Mouse Model of Cerebellar Neurodegeneration" . Neurotherapeutics . 18 (3): 1748– 1767. doi :10.1007/s13311-021-01044-3 . PMC 8609004 PMID 33829414 . ^ Pérez-Martín, Ester; Pérez-Revuelta, Laura; Barahona-López, Cristina; Pérez-Boyero, David; Alonso, José R.; Díaz, David; Weruaga, Eduardo (January 2023). "Oleoylethanolamide Treatment Modulates Both Neuroinflammation and Microgliosis, and Prevents Massive Leukocyte Infiltration to the Cerebellum in a Mouse Model of Neuronal Degeneration" . International Journal of Molecular Sciences . 24 (11): 9691. doi :10.3390/ijms24119691 ISSN 1422-0067 . PMC 10253688 PMID 37298639 .
External links
Receptor (ligands )
CB1 Tooltip Cannabinoid receptor type 1
Agonists(abridged,full list ) Inverse agonists Antagonists
CB2 Tooltip Cannabinoid receptor type 2
Agonists
2-AG 2-AGE (noladin ether) 3,3'-Diindolylmethane 4-O-Methylhonokiol α-Amyrin · β-Amyrin A-796,260 A-834,735 A-836,339 AM-1172 AM-1221 AM-1235 AM-1241 AM-2232 Anandamide AZ-11713908 Cannabinol Caryophyllene CB-13 CBS-0550 CP 55,940 GW-405,833 (L-768,242) GW-842,166X HU-308 JTE 7-31 JWH-007 JWH-015 JWH-018 JWH-73 JWH-133 L-759,633 L-759,656 Lenabasum (anabasum) Magnolol MDA-19 Nabitan NADA Olorinab (APD-371) PF-03550096 S-444,823 SER-601 Serinolamide A UR-144 Tedalinab THC (dronabinol) THCV Tetrahydromagnolol Virodhamine Antagonists
NAGly GPR18 )
GPR55
GPR119
Transporter (modulators )
eCBTs Tooltip Endocannabinoid transporter
Enzyme (modulators )
Others
Others: 2-PG (directly potentiates activity of 2-AG at CB1 receptor) ARN-272 (FAAH-like anandamide transporter inhibitor)
PPARα Tooltip Peroxisome proliferator-activated receptor alpha PPARδ Tooltip Peroxisome proliferator-activated receptor delta PPARγ Tooltip Peroxisome proliferator-activated receptor gamma Non-selective
TRPA
Activators
4-Hydroxynonenal 4-Oxo-2-nonenal 5,6-EET 12S-HpETE 15-Deoxy-Δ12,14 -prostaglandin J2 α-Sanshool (ginger , Sichuan and melegueta peppers )Acrolein Allicin (garlic )Allyl isothiocyanate (mustard , radish , horseradish , wasabi )AM404 ASP-7663 Bradykinin Cannabichromene (cannabis )Cannabidiol (cannabis )Cannabigerol (cannabis )Cinnamaldehyde (cinnamon )CR gas (dibenzoxazepine; DBO) CS gas (2-chlorobenzal malononitrile) Cuminaldehyde (cumin )Curcumin (turmeric )Dehydroligustilide (celery )Diallyl disulfide Dicentrine (Lindera Farnesyl thiosalicylic acid Formalin Gingerols (ginger )Hepoxilin A3 Hepoxilin B3 Hydrogen peroxide Icilin Isothiocyanate JT-010 Ligustilide (celery , Angelica acutiloba Linalool (Sichuan pepper , thyme )Methylglyoxal Methyl salicylate (wintergreen )N-Methylmaleimide Nicotine (tobacco )Oleocanthal (olive oil )Paclitaxel (Pacific yew )Paracetamol (acetaminophen) PF-4840154 Phenacyl chloride Polygodial (Dorrigo pepper )Shogaols (ginger , Sichuan and melegueta peppers )Tear gases Tetrahydrocannabinol (cannabis )Tetrahydrocannabiorcol Thiopropanal S-oxide (onion )Umbellulone (Umbellularia californica )WIN 55,212-2 Blockers
TRPC
TRPM
TRPML
TRPP
TRPV
Activators
2-APB 5,6-EET 9-HODE 9-oxoODE 12S-HETE 12S-HpETE 13-HODE 13-oxoODE 20-HETE α-Sanshool (ginger , Sichuan and melegueta peppers )Allicin (garlic )AM404 Anandamide Bisandrographolide (Andrographis paniculata Camphor (camphor laurel , rosemary , camphorweed , African blue basil , camphor basil )Cannabidiol (cannabis )Cannabidivarin (cannabis )Capsaicin (chili pepper )Carvacrol (oregano , thyme , pepperwort , wild bergamot , others)DHEA Diacyl glycerol Dihydrocapsaicin (chili pepper )Estradiol Eugenol (basil , clove )Evodiamine (Euodia ruticarpa Gingerols (ginger )GSK1016790A Heat Hepoxilin A3 Hepoxilin B3 Homocapsaicin (chili pepper )Homodihydrocapsaicin (chili pepper )Incensole (incense )Lysophosphatidic acid Low pH (acidic conditions)
Menthol (mint )N-Arachidonoyl dopamine N-Oleoyldopamine N-Oleoylethanolamide Nonivamide (PAVA) (PAVA spray )Nordihydrocapsaicin (chili pepper )Paclitaxel (Pacific yew )Paracetamol (acetaminophen) Phenylacetylrinvanil Phorbol esters (e.g., 4α-PDD )Piperine (black pepper , long pepper )Polygodial (Dorrigo pepper )Probenecid Protons RhTx Rutamarin (Ruta graveolens Resiniferatoxin (RTX) (Euphorbia resinifera /pooissonii Shogaols (ginger , Sichuan and melegueta peppers )Tetrahydrocannabivarin (cannabis )Thymol (thyme , oregano )Tinyatoxin (Euphorbia resinifera /pooissonii Tramadol Vanillin (vanilla )Zucapsaicin Blockers