|
Copper(II) arsenate
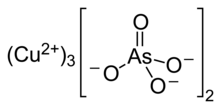
Copper arsenate (Cu3(AsO4)2·4H2O, or Cu5H2(AsO4)4·2H2O), also called copper orthoarsenate, tricopper arsenate, cupric arsenate, or tricopper orthoarsenate, is a blue or bluish-green powder insoluble in water and alcohol and soluble in aqueous ammonium and dilute acids. Its CAS number is or . UsesCopper arsenate is an insecticide used in agriculture. It is also used as a herbicide, fungicide, and a rodenticide. It is also used as a poison in slug baits. Copper arsenate can also be a misnomer for copper arsenite, especially when meant as a pigment. Natural occurrencesAnhydrous copper arsenate, Cu3(AsO4)2, is found in nature as the mineral lammerite.[3] Copper arsenate tetrahydrate, Cu3(AsO4)2·4H2O, occurs naturally as the mineral rollandite.[4] Related compoundsCopper arsenate hydroxide or basic copper arsenate (Cu(OH)AsO4) is a basic variant with CAS number . It is found naturally as the mineral olivenite. It is used as an insecticide, fungicide, and miticide. Its use is banned in Thailand since 2001.[5] See also
References
External linksWikimedia Commons has media related to Copper(II) arsenate. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||