|
Selurampanel
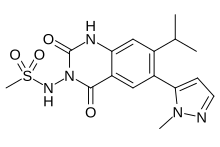
Selurampanel (INN, code name BGG492) is a drug closely related to the quinoxalinedione series which acts as a competitive antagonist of the AMPA and kainate receptors and, as of 2015, is being investigated in clinical trials by Novartis for the treatment of epilepsy.[1][2][3] It has also been studied in the acute treatment of migraine, and was found to produce some pain relief, but with a relatively high rate of side effects.[4] References
|
||||||||||||||||||||||||||||||||