|
Prorenone
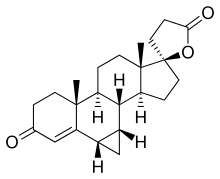
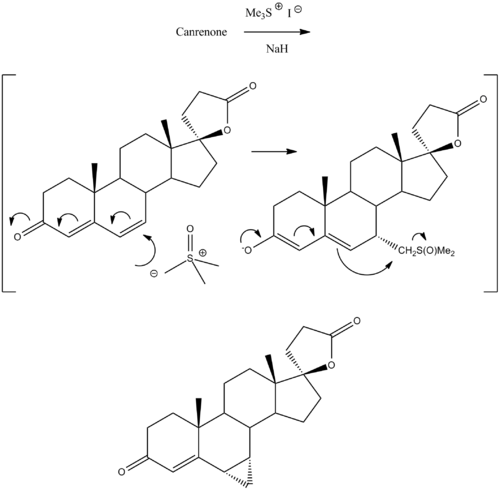
Prorenone (developmental code name SC-23133) is a steroidal antimineralocorticoid of the spirolactone group related to spironolactone that was never marketed.[1] It is the lactonic form of prorenoic acid (prorenoate), and prorenoate potassium (SC-23992), the potassium salt of prorenoic acid, also exists.[1] Prorenoate potassium is about 8 times more potent than spironolactone as an antimineralocorticoid in animals, and it may act as a prodrug to prorenone.[1] In addition to the mineralocorticoid receptor, prorenone also binds to the glucocorticoid, androgen, and progesterone receptors.[2][3] The antiandrogenic potency of prorenone in vivo in animals is close to that of spironolactone.[3] Similarly to spironolactone, prorenone is also a potent inhibitor of aldosterone biosynthesis.[4] ChemistrySynthesisProrenone can be synthesized via a Johnson–Corey–Chaykovsky reaction by reaction of canrenone with trimethylsulfoxonium iodide and sodium hydride.[5] See alsoReferences
|
||||||||||||||||||||||||||||||||||