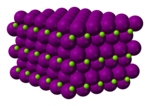
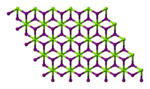
Magnesium iodide
Names
IUPAC name
Magnesium iodide
Identifiers
ChemSpider
ECHA InfoCard 100.030.738
EC Number
UNII
InChI=1S/2HI.Mg/h2*1H;/q;;+2/p-2
N Key: BLQJIBCZHWBKSL-UHFFFAOYSA-L
N InChI=1/2HI.Mg/h2*1H;/q;;+2/p-2
Key: BLQJIBCZHWBKSL-NUQVWONBAV
Properties
MgI2 (anhydrous)MgI2 ·6H2 O (hexahydrate)MgI2 ·8H2 O (octahydrate)[ 1]
Molar mass
278.1139 g/mol (anhydrous) 386.2005 g/mol (hexahydrate) 422.236 g/mol (octahydrate)
Appearance
white crystalline solid
Odor
odorless
Density
4.43 g/cm3 (anhydrous solid) 2.353 g/cm3 (hexahydrate solid) 2.098 g/cm3 (octahydrate solid)
Melting point
637 °C (1,179 °F; 910 K) (anhydrous, decomposes)
54.7 g/(100 cm3 ) (anhydrous, 0 °C) 148 g/(100 cm3 ) (anhydrous, 18 °C)[ 2] 81 g/(100 cm3 ) (octahydrate, 20 °C)
Solubility
soluble in ether , alcohol and ammonia
−111.0·10−6 cm3 /mol
Structure
Thermochemistry
74 J/(mol·K)
134 J/(mol·K)
−364 kJ/mol
Hazards
GHS labelling
Warning
H315 , H319
NFPA 704
Related compounds
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
Chemical compound
Magnesium iodide is an inorganic compound with the chemical formula Mg I 2 hydrates MgI2 ·x H2 O . Magnesium iodide is a salt of magnesium and hydrogen iodide . These salts are typical ionic halides , being highly soluble in water.
Uses
Magnesium iodide has few commercial uses, but can be used to prepare compounds for organic synthesis .
Preparation
Magnesium iodide can be prepared from magnesium oxide , magnesium hydroxide , and magnesium carbonate by treatment with hydroiodic acid :[ 3]
MgO + 2 HI → MgI2 + H2 O Mg(OH)2 + 2 HI → MgI2 + 2 H2 OMgCO3 + 2 HI → MgI2 + CO2 + H2 O
Reactions
Magnesium iodide is stable at high heat under a hydrogen atmosphere, but decomposes in air at normal temperatures, turning brown from the release of elemental iodine . When heated in air, it decomposes completely to magnesium oxide.[ 4]
Another method to prepare MgI2 is mixing powdered elemental iodine and magnesium metal . In order to obtain anhydrous MgI2 , the reaction should be conducted in a strictly anhydrous atmosphere; dry-diethyl ether can be used as a solvent.
Usage of magnesium iodide in the Baylis-Hillman reaction tends to give (Z vinyl compounds.[ 5]
Demethylation of certain aromatic methyl ethers can be afforded using magnesium iodide in diethyl ether .[ 6]
Hydrates
Two hydrates are known, the octahydrate and the nonahydrate, both verified by X-ray crystallography These hydrates feature [Mg(H2 O)6 ]2+ ions.[ 7]
References
^
Perry, Dale L.; Phillips, Sidney L. (1995), Handbook of Inorganic Compounds ISBN 0-8493-8671-3 , retrieved 2007-12-09
^ Magnesium Iodide MSDS at AlfaAesar [permanent dead link ] ^
Patnaik, Pradyot (2003), Handbook of Inorganic Chemicals ISBN 0-07-049439-8 , retrieved 2007-12-09
^
Wilsmore, N. T. M. (1891). "Note on Magnesium Iodide" . In James Hector (ed.). Report of the Third Meeting of the Australasian Association for the Advancement of Science . Sydney: The Association. p. 116. Retrieved 2007-12-09 .
^
Tietze, Lutz-Friedjan; Brasche, Gordon; Gericke, Kersten (2006), "Domino Reactions in Organic Synthesis" , Chemical Reviews , 96 (1), Wiley-VCH: 115–136, doi :10.1021/cr950027e , ISBN 3-527-29060-5 PMID 11848746 , retrieved 2007-12-09
^ Yamaguchi, Seiji; Nedachi, Masahiro; Yokoyama, Hajime; Hirai, Yoshiro (October 1999). "Regioselective demethylation of 2,6-dimethoxybenzaldehydes with magnesium iodide etherate". Tetrahedron Letters . 40 (41): 7363–7365. doi :10.1016/S0040-4039(99)01411-2 . ^ Hennings, Erik; Schmidt, Horst; Voigt, Wolfgang (2013). "Crystal Structures of Hydrates of Simple Inorganic Salts. I. Water-Rich Magnesium Halide Hydrates MgCl2 ·8H2 O, MgCl2 ·12H2 O, MgBr2 ·6H2 O, MgBr2 ·9H2 O, MgI2 ·8H2 O and MgI2 ·9H2 O". Acta Crystallographica Section C Crystal Structure Communications . 69 (11): 1292–1300. doi :10.1107/S0108270113028138 . PMID 24192174 .
Salts and covalent derivatives of the
iodide ion